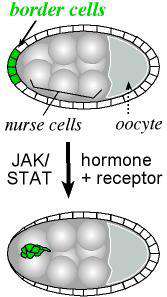
Border cells move out of the epithelium
Research interests: cell migration
In many instances, cells must migrate long distances to fulfill their destiny. For example, numerous cell types in developing embryos are born far away from where they will be required in the fully formed organism. In the case of an injury, immune cells travel to the site to fight infections, and skin cells move to repair a wound. These migrations occur with great precision in time and space and few cells get lost. As someone who needs an alarm clock to wake up and a map to get around Baltimore, I think this is amazing. How are cells selected to become motile cells, and detach from their neighbors that stay behind? How do they know when it is time to go? What signals guide their movements through complex tissues? How do they “put on the brakes” when it is time to stop? These questions are of fundamental interest to developmental biologists, and have major implications in medicine as well. Defects in cell migration can lead to birth defects in humans. In addition, cancers are most lethal when they become metastatic, acquiring the ability to leave a tumor and spread and hide throughout the body.
My research group uses Drosophila melanogaster as a model system for addressing unanswered questions in cell movement. The vast array of genetic tools in fruit flies, as well as their fast generation time and small size, makes them ideal for these studies. Also, it has been shown that genes involved in cell movements in flies also play roles in cell migration and cancer metastasis in vertebrates (reviewed in Jang et al 2007; Naora and Montell, 2005). We also collaborate to use mathematical modeling to made new, testable predictions about how cell migration works. (Peercy and Starz-Gaiano, 2020)
Specifying a migratory cell population
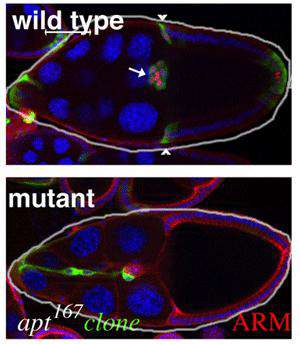
Wild type and mutant egg chambers
The current focus of the lab is on the movement of the border cells, a cluster of cells in the ovary that detach from an epithelial cell layer and undergo a stereotyped movement between germ line cells as the egg develops (schematized in Figure 1). Border cells ultimately form a structure that allows the egg to be fertilized. We have found a three-component genetic circuit of transcription factors that is responsible for specifying the correct number of motile cells. Loss or overexpression of any of the genes –apontic, stat, or slow border cells – causes too many cells to become motile, and thereby disrupts cell migration (see Figure 2 for an example of an apontic (apt) loss of function phenotype). We are interested in the downstream mechanisms responsible for this defect; in particular, what molecules cause the cells to remain attached to their neighbors when they should disconnect? Regulation of cell adhesion molecules such as Cadherins are likely to be involved in this detachment step, and this is being investigated.
Steroid hormone control of timing
Border cells are specified significantly before they begin to migrate. As is the case for humans, steroid hormones in insects are responsible for coordinating developmental events over time, and it has been shown that a steroid hormone signal gives the border cells a cue to go now. There is only one steroid hormone in flies, called ecdysone. Like other hormones, ecdysone binds to a receptor, which translocates to the nucleus to initiate a cascade of transcription. Ecdysone receptor acts in a complex with several required co-factors including Ultraspiracle, the fly homolog to human RXR, and Taiman, which shares homology with the breast cancer oncogene amplified in breast cancer 1 (AIB1/SRC-3). All of these genes are required for border cell migration (Bai, 2000). We are very interested in the downstream signals that instruct border cells to “go”. Our recent work suggests this one steroid hormone orchestrates cell polarity, motility, and cell adhesion (Bhattacharya and Starz-Gaiano, 2024). Loss of function and gain of function studies are underway to understands critical target genes better. Required genes may likely play a role in breast and ovarian cancer metastases.

Chemical-based navigation
Our recent work aims to understand how cells use chemical information in their environment to navigate to new places. Chemicals that cells move towards are called chemoattractants. We used a combination of genetic mutants, live imaging, advanced microscopy, and mathematical modeling to show that migrating cells have to adapt to changing concentrations of chemoattractants (George et al, 2025). We plan to continue to use similar techniques to determine what precise information cells “see” in their environment, how this information is distributed effectively, and how cells adapt to the signals they are given to get through uneven terrains of the developing ovary. This work can help us understand how cells migrate in many organisms; for example, how cells respond to tissue damage and infections in humans.
References:
1 Jang AC, Starz-Gaiano M, Montell DJ. (2007) Modeling migration and metastasis in Drosophila. J Mammary Gland Biol Neoplasia. Sep;12(2-3):103-14.
2 Naora H, Montell DJ. (2005) Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. May;5(5):355-66.
3 Peercy, B. and Starz-Gaiano, M. (2020) Clustered Cell Migration: Modeling the Model System of Drosophila Border Cells. Sem in Cell and Dev Bio. Apr; 100:167-176. doi: 10.1016/j.semcdb.2019.11.010.
4. Bai J, Uehara Y, Montell DJ. (2000) Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000 Dec 22;103(7):1047-58.
5. Bhattacharya M, and Starz-Gaiano M. (2024) Steroid hormone signaling synchronizes cell migration machinery, adhesion, and polarity to direct collective movement. Journal of Cell Science. 137, jcs261164. doi:10.1242/jcs.261164
6. George A, Akhavan N, Peercy BE, Starz-Gaiano M. (2025) Chemotaxis of Drosophila border cells is modulated by tissue geometry through dispersion of chemoattractants. iScience. Feb 5;28(3):111959. doi: 10.1016/j.isci.2025.111959